3 months ago Category :
The regulation of medical devices is of utmost importance to ensure patient safety and effectiveness in healthcare practices. Various calculation tools play a crucial role in assessing the compliance of these devices with regulatory standards. Let's delve into how these tools contribute to the regulation of medical devices.
Read More →3 months ago Category :
Business Resilience Strategies in the Russian Healthcare System
Read More →3 months ago Category :
In today's fast-paced and ever-changing business landscape, having resilient strategies in place is crucial for organizations to navigate challenges and thrive. This is especially true in industries like medical devices, where regulations play a key role in shaping the market environment. Understanding and effectively managing medical devices regulation is a critical component of building business resilience in this sector.
Read More →3 months ago Category :
Business Resilience Strategies for Healthy Fast Food Restaurants
Read More →3 months ago Category :
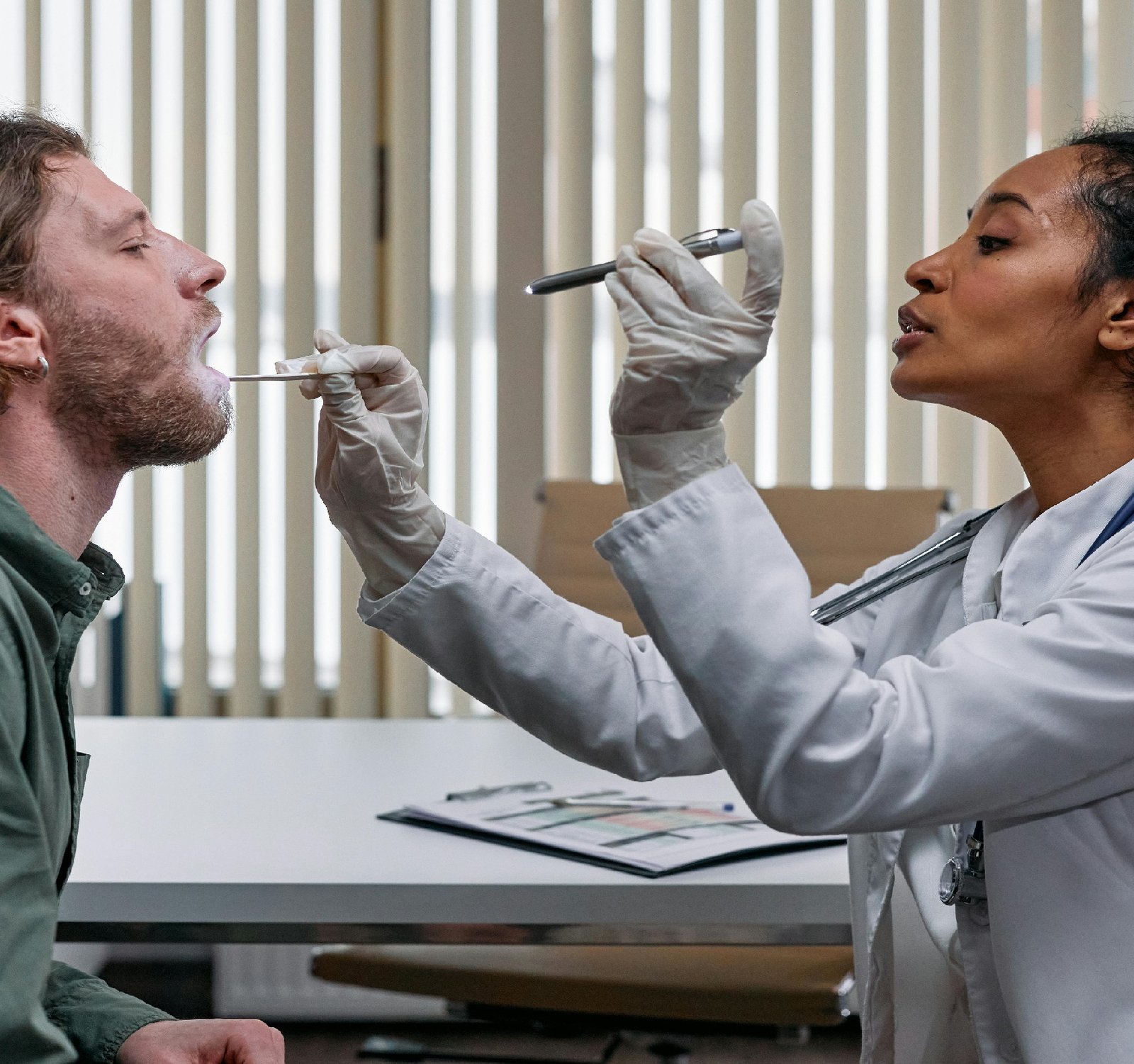
The Russian healthcare system has been a topic of interest and discussion for many years. With a population of over 146 million people, Russia faces challenges in providing adequate healthcare services to its citizens. The country’s business planning in the healthcare sector plays a key role in addressing these challenges and shaping the future of healthcare delivery in Russia.
Read More →3 months ago Category :
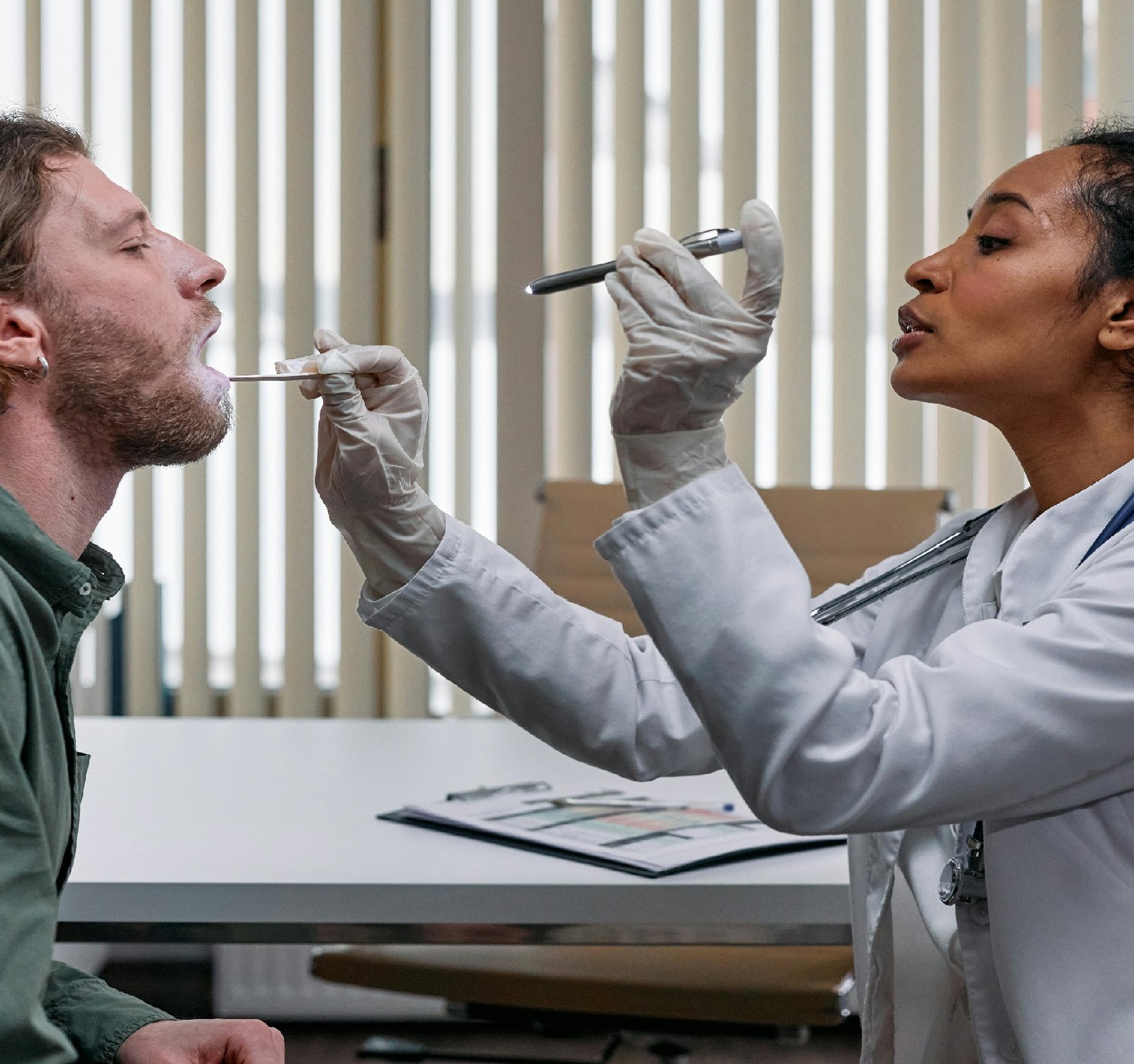
Navigating Regulatory Compliance in Business Planning for Medical Devices
Read More →3 months ago Category :
"The Rise of Healthy Fast Food: How Business Planning is Revolutionizing the Industry"
Read More →3 months ago Category :
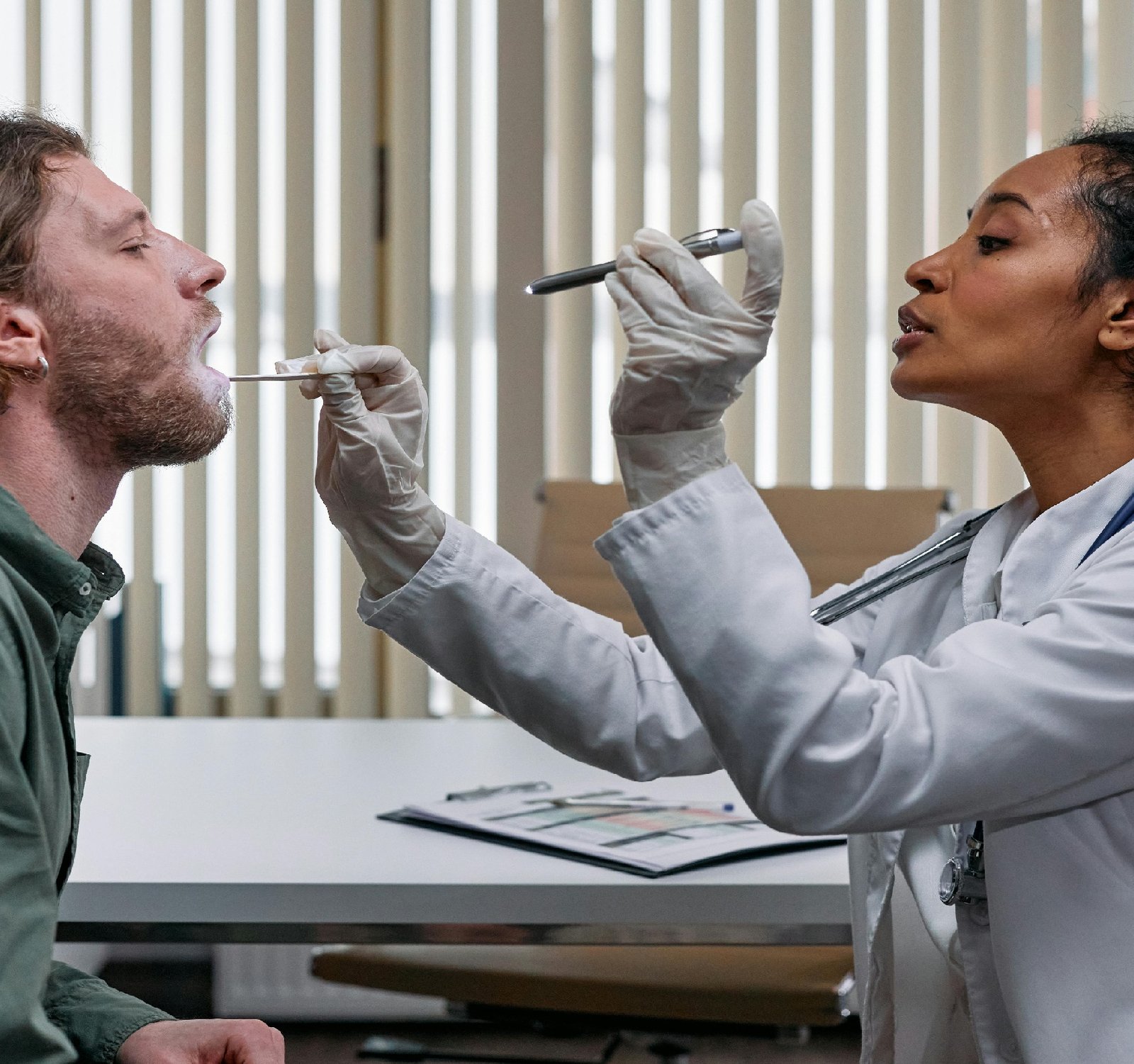
The healthcare system in Russia is subject to various legal compliance requirements that businesses operating in this sector must adhere to. From licensing and accreditation to data protection and consumer rights, there are numerous laws and regulations that govern the provision of healthcare services in the country.
Read More →3 months ago Category :
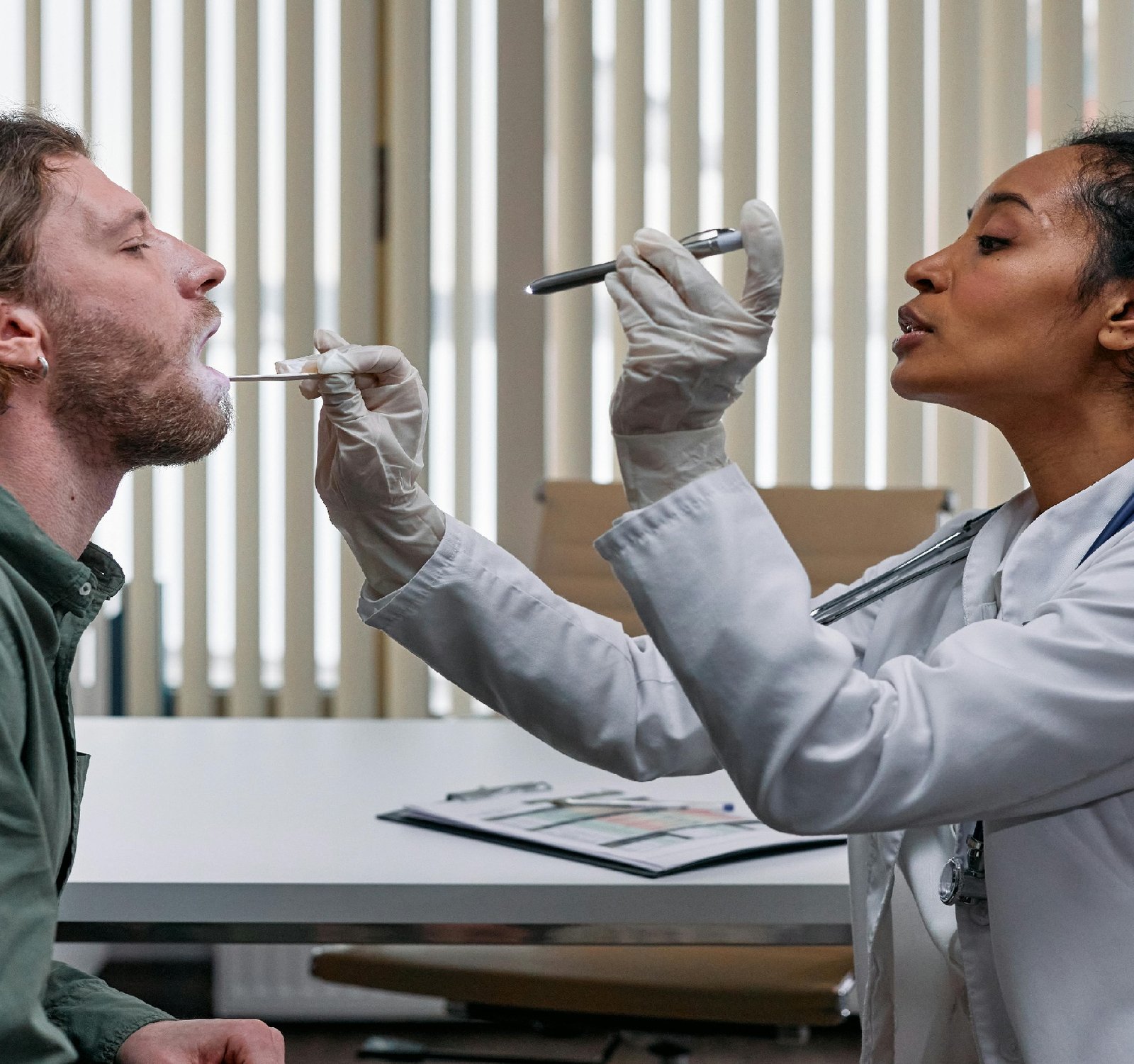
Navigating Legal Compliance in the Medical Devices Industry
Read More →3 months ago Category :
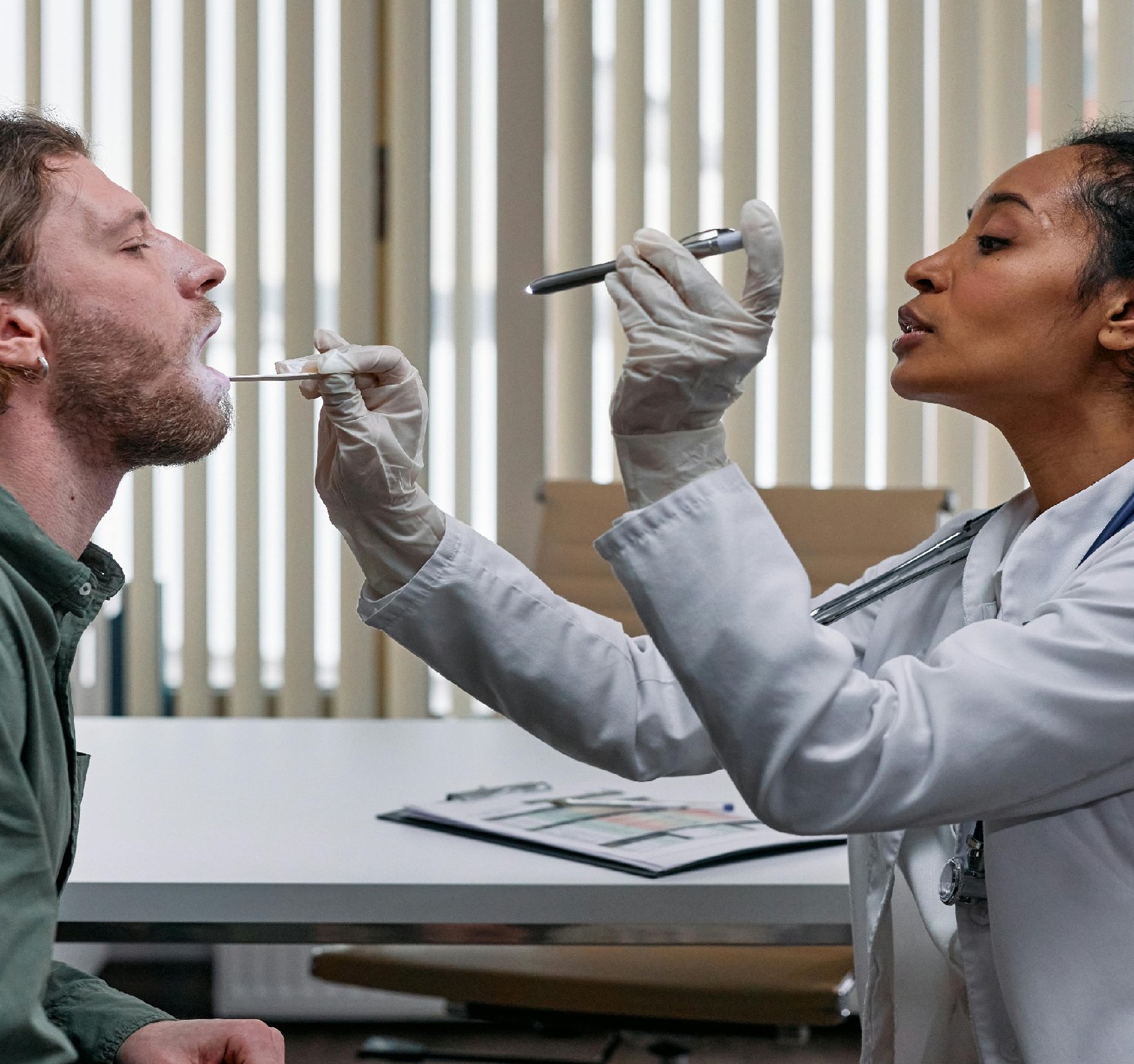
Medical devices play a crucial role in modern healthcare, providing essential tools for diagnosis, treatment, and monitoring of medical conditions. However, the regulation of medical devices is a complex and evolving field that presents challenges for both manufacturers and regulatory authorities. In this blog post, we will explore the key aspects of medical devices regulation and how businesses can navigate this landscape to successfully kick off their operations in the medical devices industry.
Read More →SEARCH
Recent News
- Zurich, Switzerland has long been known for its exceptional quality of life, beautiful surroundings, and high standard of healthcare. In contrast, the Russian healthcare system has faced various challenges and struggles over the years. Let's delve into the differences between the healthcare systems in Zurich, Switzerland, and Russia.
- Navigating Medical Device Regulations in Zurich, Switzerland
- In the bustling city of Zurich, Switzerland, finding healthy fast food options can be a challenge. However, with a little exploration and curiosity, you can discover some fantastic spots that offer nutritious and delicious meals on the go.
- YouTube Content Creation: Exploring the Russian Healthcare System
- In today's digital age, YouTube has become a powerful platform for content creators to share their knowledge and expertise with a global audience. One particular niche that has been gaining traction on YouTube is the creation and translation of content related to medical devices regulation.
- Are you looking for tips on creating YouTube content about healthy fast food options and the importance of translation in reaching a wider audience? Let's dive into how you can combine these two aspects to create engaging and informative videos for your channel.
- Exploring the Russian Healthcare System: Insights from a YouTube Channel
- Navigating the Regulatory Landscape for Medical Devices on YouTube